Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Advancing Diagnostic Accuracy and Efficiency through Machine Learning Integration in Healthcare
Authors: Prof. Priyanka Bhore, Utkarsh Kumar, Aniket Ovhal, Rahul Talvar, Nikhil Waghmare
DOI Link: https://doi.org/10.22214/ijraset.2025.66707
Certificate: View Certificate
Abstract
Machine learning (ML) has the potential to transform healthcare by improving the accuracy and efficiency of medical diagnoses. This project showcases the use of ML in healthcare through a DenseNet121 model designed to classify chest X-ray images into four categories: Pneumonia, Atelectasis, Pneumothorax, and No Finding. Utilizing the DenseNet121 architecture, recognized for its strong feature extraction abilities, the model was trained on a dataset of chest X-ray images along with relevant metadata. The goal was to accurately identify these conditions, thereby assisting healthcare professionals in their clinical decision-making. During the training phase, the model\'s performance was closely monitored with real-time validation to avoid overfitting. After training, the model\'s predictions on the test set were assessed using ROC curves and AUC scores, showing strong performance in classifying the targeted conditions. Incorporating ML models like DenseNet121 into clinical environments can greatly improve diagnostic accuracy and efficiency. These models can collaborate with hospitals to deliver quick and dependable diagnoses, alleviating some of the pressures on healthcare professionals and potentially leading to improved patient outcomes. The successful application of ML in this project underscores its promise as a valuable asset in the evolution of healthcare.
Introduction
I. INTRODUCTION
The use of machine learning (ML) in healthcare could transform how we approach medical diagnosis, treatment, and patient care. ML algorithms, especially those based on deep learning, are capable of analyzing large datasets and recognizing patterns that might go unnoticed by humans. This ability positions ML as a valuable asset in improving diagnostic accuracy and efficiency, which can result in enhanced patient outcomes.
In this project, we aim to showcase how machine learning can be applied in healthcare by utilizing a DenseNet121 model to classify chest X-ray images. Chest X-rays serve as a widely used diagnostic tool for identifying various issues related to the lungs and thoracic cavity. The accurate and prompt interpretation of these images is essential for the effective management and treatment of conditions like Pneumonia, Atelectasis,andPneumothorax.
The DenseNet121 model, a deep convolutional neural network, excels at extracting detailed features from images thanks to its unique dense connectivity pattern. By utilizing pre-trained weights from ImageNet, we can adapt the model for our specific needs, taking advantage of the general visual features learned from a vast and varied dataset. This transfer learning strategy allows us to achieve high accuracy even with a smaller labeled dataset (1, 5, 8).
Our approach consists of several important steps
- Data Preparation: We start by preprocessing the dataset, which includes mapping image paths, cleaning up labels, and creating binary columns for each target condition. The data is then divided into training, validation, and test sets, ensuring that class distribution is maintained through stratification.
- Custom Data Generator: We implement a custom data generator to manage image loading and preprocessing in batches, which helps optimize data handling and reduce memory usage.
- Model Design and Training: The DenseNet121 model is constructed and trained using the preprocessed dataset. We monitor the training process with real-time validation to avoid overfitting.
- Evaluation: We assess the model’s performance using ROC curves and AUC scores, which provide a thorough evaluation of its classification abilities (4, 10).
By integrating machine learning models like DenseNet121 into clinical workflows, healthcare professionals can enhance diagnostic accuracy and efficiency. These models can collaborate with hospitals, offering quick and reliable diagnoses, alleviating the workload on healthcare staff, and potentially improving patient outcomes (2, 3, 6). This project underscores the promise of machine learning in healthcare, demonstrating how sophisticated algorithms can bolster and refine traditional diagnostic practices. Through partnerships with healthcare institutions, machine learning can significantly enhance the quality of care for patients, ensuring timely and precise diagnoses, and ultimately revolutionizing the healthcare landscape.
II. METHODS AND MATERIAL
A. Dataset Description
The dataset utilized in this project consists of a collection of chest X-ray images, accompanied by metadata that includes patient demographics and diagnostic labels. It was sourced from a publicly accessible repository, making it a valuable asset for the development and testing of machine learning models aimed at medical image classification.
- Source: The dataset can be found at NIH Chest X-Rays / NIH Chest X-rays. https://www.kaggle.com/datasets/nih-chest-xrays/data
- Content: This dataset features thousands of chest X-ray images along with metadata such as patient ID, age, gender, and diagnostic labels.
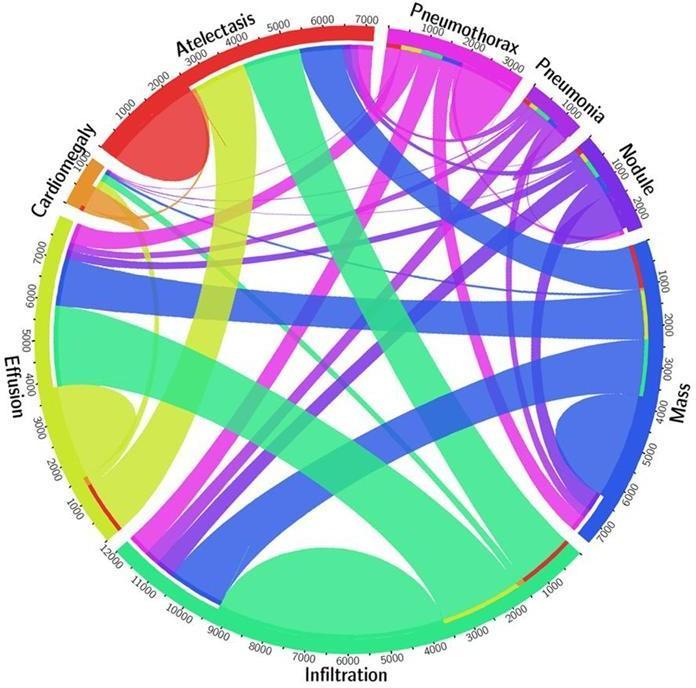
Fig. Data Visualization
B. Data Preprocessing
Effective data preprocessing is essential for maintaining the quality and consistency of the dataset. The following steps were implemented to prepare the data:
- Mapping Image Paths: Image paths were organized to facilitate easy access during model training. Each image filename was associated with its respective path in the dataset. •
- Patient Data Processing: Patient information was refined to enhance data quality. Age values were capped at 100 to eliminate unrealistic data points.
- Label Sanitization: Diagnostic labels were sanitized to ensure consistency. Labels such as 'No Finding' were converted to 'No_Finding' to avoid issues with spaces in column names.
- Binary Columns Creation: Binary columns were created for each target condition (Pneumonia, Atelectasis, Pneumothorax, and No Finding). This facilitated multi-label classification by indicating.
- Data Filtering: The dataset was refined to include only images that matched the chosen conditions. This filtered data was subsequently divided into training, validation, and test sets, ensuring stratification to maintain the distribution of classes.
C. Custom Data Generator
A custom data generator was created to manage image loading and preprocessing in batches. This method ensured efficient data handling while reducing memory usage during model training.
- Implementation: The custom data generator processed images in batches, resizing them to the necessary input size of 224x224 pixels and normalizing them for the DenseNet121 model.
- Efficiency: By processing data in batches, the generator effectively managed memory and handled data, making it ideal for large datasets.
D. Model Design
The DenseNet121 architecture was chosen for its strong feature extraction abilities (5, 8). The model underwent fine-tuning with pre-trained weights sourced from ImageNet.
- Architecture: The DenseNet121 model was constructed using pre-trained weights from ImageNet, omitting the top layer. A global average pooling layer was incorporated to consolidate feature maps, followed by a dense layer utilizing sigmoid activation for multi-label classification (6).
- Compiler Settings: The model was compiled with the Adam optimizer and binary cross-entropy loss, which is suitable for multi-label classification tasks (7).
E. Training Procedure
The training environment made use of Kaggle's GPU resources along with the Mirrored Strategy to enable distributed training across four GPUs.
- Training procedure: The training process involved utilizing Kaggle's GPU resources and the Mirrored Strategy for effective distributed training. The model underwent training for 20 epochs, incorporating real-time validation to keep track of performance and avoid overfitting. A dummy input was employed to construct the model and verify the compatibility of input shapes prior to the training phase.
F. Evaluation Metrics
The model's performance was assessed through various metrics to ensure a thorough evaluation of its classification abilities (3, 10).
- ROC Curve: ROC curves were utilized to illustrate the model's performance and determine AUC scores for each class (1).
- Confusion Matrix and Classification Report: Other evaluation metrics, including the confusion matrix and classification report, were employed to measure classification accuracy and overall performance (2).
- Label Distribution Check: Checks were conducted to confirm that each class in the test set was adequately represented, addressing any potential data imbalance issues (8).
III. RESULTS AND DISCUSSION
A. Results
1) Model Performance
The DenseNet121 model was trained using a chest X-ray dataset to categorize images into four groups: Pneumonia,
Atelectasis, Pneumothorax, and No Finding. It achieved a training accuracy of 0.9629 with a training loss of 0.0971. For validation, the binary accuracy was 0.7831, and the validation loss stood at 0.4541. The distribution of labels in the test set was as follows: Pneumonia (2633), Atelectasis (2285), Pneumothorax (3943), and No Finding (2871)
2) ROC Curves and AUC Scores
The ROC curves offered a clear visual representation of how well the model performed for each class. We plotted the True Positive Rate (sensitivity) against the False Positive Rate (1 - specificity) across different threshold settings.
The AUC scores reflected the model's effectiveness in distinguishing between the classes:
-Pneumonia: AUC = 0.79 (Blue line)
-Atelectasis: AUC = 0.82 (Green line)
-Pneumothorax: AUC = 0.84 (Orange line).
3) Confusion Matrix and Classification Report
The confusion matrix and classification report offered additional insights into the model's performance, detailing the true positive, false positive, true negative, and false negative rates for each class.
The high AUC scores for Pneumonia and No Finding demonstrate strong diagnostic performance for these categories, whereas the AUC score for Pneumothorax indicates moderate performance.
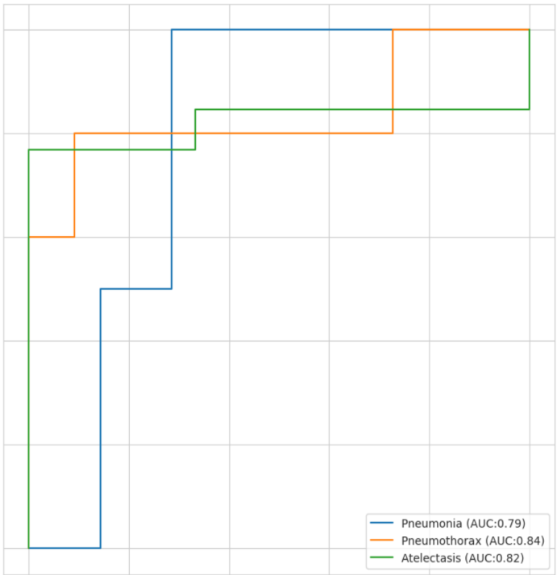
Fig. AUC CURVE
4) Project Github Link
To Test the Model, You have to download whole repository and run the run_flask_app.py python file in a compiler. The code is made in such a manner that all necessary libraries will be installed automatically. The user interface will pop up in a browser using an IP Address:127.0.0.1 on Port No. 5000.
The Web Application is based on Flask Environment. And trained model is present with the name chest_xray_model.h5 https://github.com/DevoRepo/Multi-Chest-X-Ray- Diagnosis-Tool
B. Discussions
- Model Strengths: The DenseNet121 architecture successfully extracted important features from chest X-ray images, resulting in precise classification of the chosen conditions (5, 8). Utilizing pre-trained weights from ImageNet enabled transfer learning, which allowed the model to leverage general visual features acquired from a vast and varied dataset (7). The custom data generator played a crucial role in efficient data management and preprocessing, enhancing the model's overall performance (6)
- Challenges and Limitations: The AUC score for Atelectasis couldn't be determined because y_true contained only one class. This underscores the need for adequate class representation in the test set (9). Data imbalance in the dataset was a significant challenge, especially,for conditions with fewer samples. Tackling this imbalance through data augmentation or obtaining additional samples could enhance model performance (2). Initial efforts to use Kaggle's TPU resources encountered limitations due to TPU usage restrictions, leading to a transition to GPU resources for training (4)
- Future Directions: Improving the dataset by adding more samples and varying the conditions can enhance the model's performance and its ability to generalize (3).Investigating advanced data augmentation methods can help tackle data imbalance and strengthen the model's robustness (6). Running real-world trials and incorporating the model into clinical workflows can offer valuable insights for refining both the model and its applications (10).
- Impact on Healthcare: The effective use of the DenseNet121 model for classifying chest X-rays highlights how machine learning can improve diagnostic accuracy and efficiency in the healthcare sector. By partnering with hospitals and healthcare professionals, these models can deliver quick and dependable diagnoses, alleviating the workload on medical staff and possibly resulting in improved patient outcomes. Working together, machine learning researchers and healthcare institutions can foster innovation and enhance the quality of care for patients. (5).
- Improved Diagnosis and Efficiency: Implementing a machine learning model in the diagnosis of chest X-ray diseases can greatly enhance both the accuracy of diagnoses and the efficiency of the process. By alleviating some of the workload from radiologists, these models allow them to concentrate on more complex cases. They can quickly assess chest X-rays to prioritize urgent cases, ensuring that high-risk patients receive timely care. Furthermore, these models can speed up the generation of reports and offer essential support in areas with a shortage of radiologists, particularly in developing countries such as India, China, and Russia. By delegating routine tasks to machine learning models, radiologists can enjoy a better work-life balance, which helps reduce burnout and increases job satisfaction, ultimately leading to improved patient outcomes and healthcare delivery.
Working Presentation
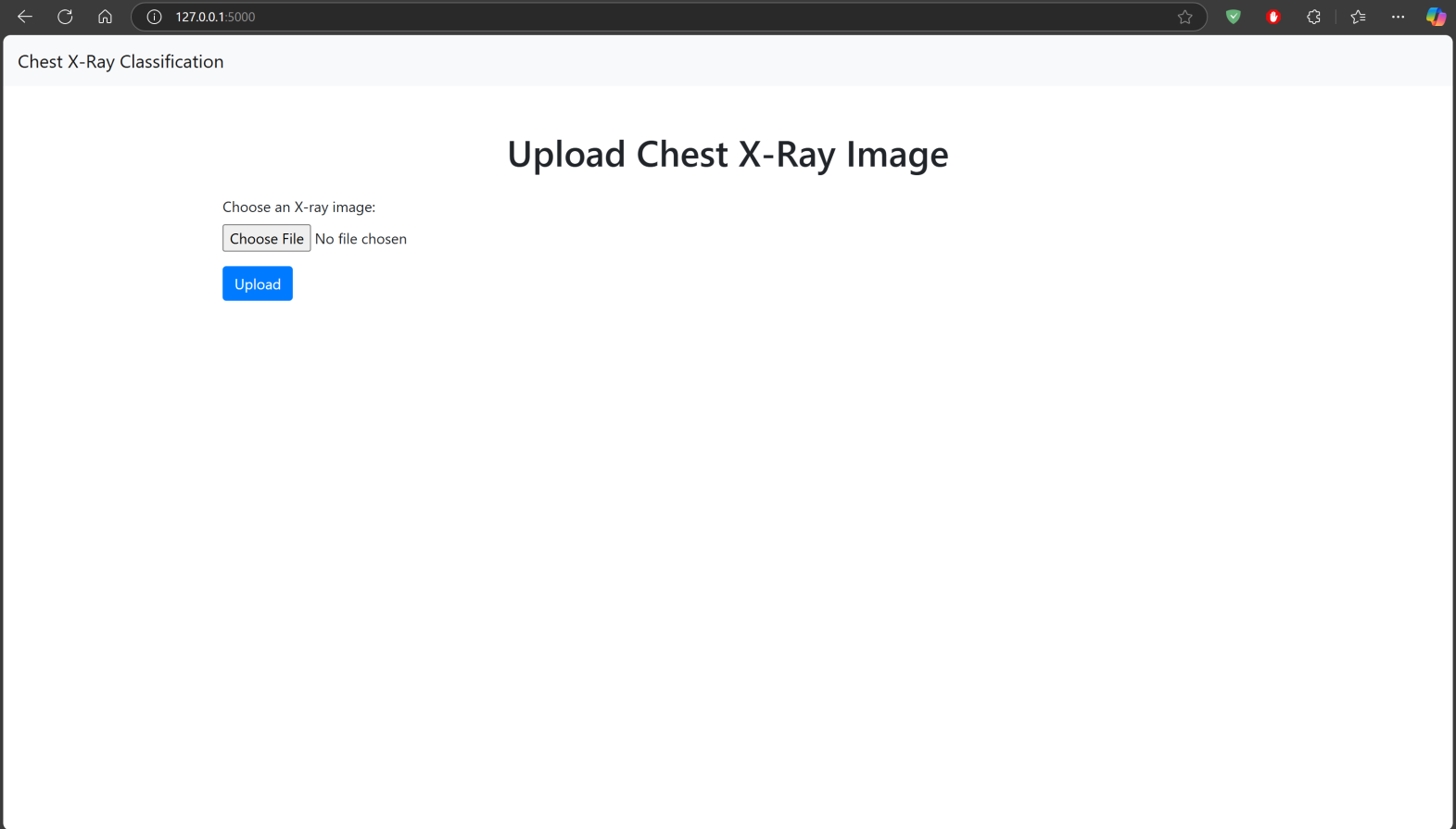
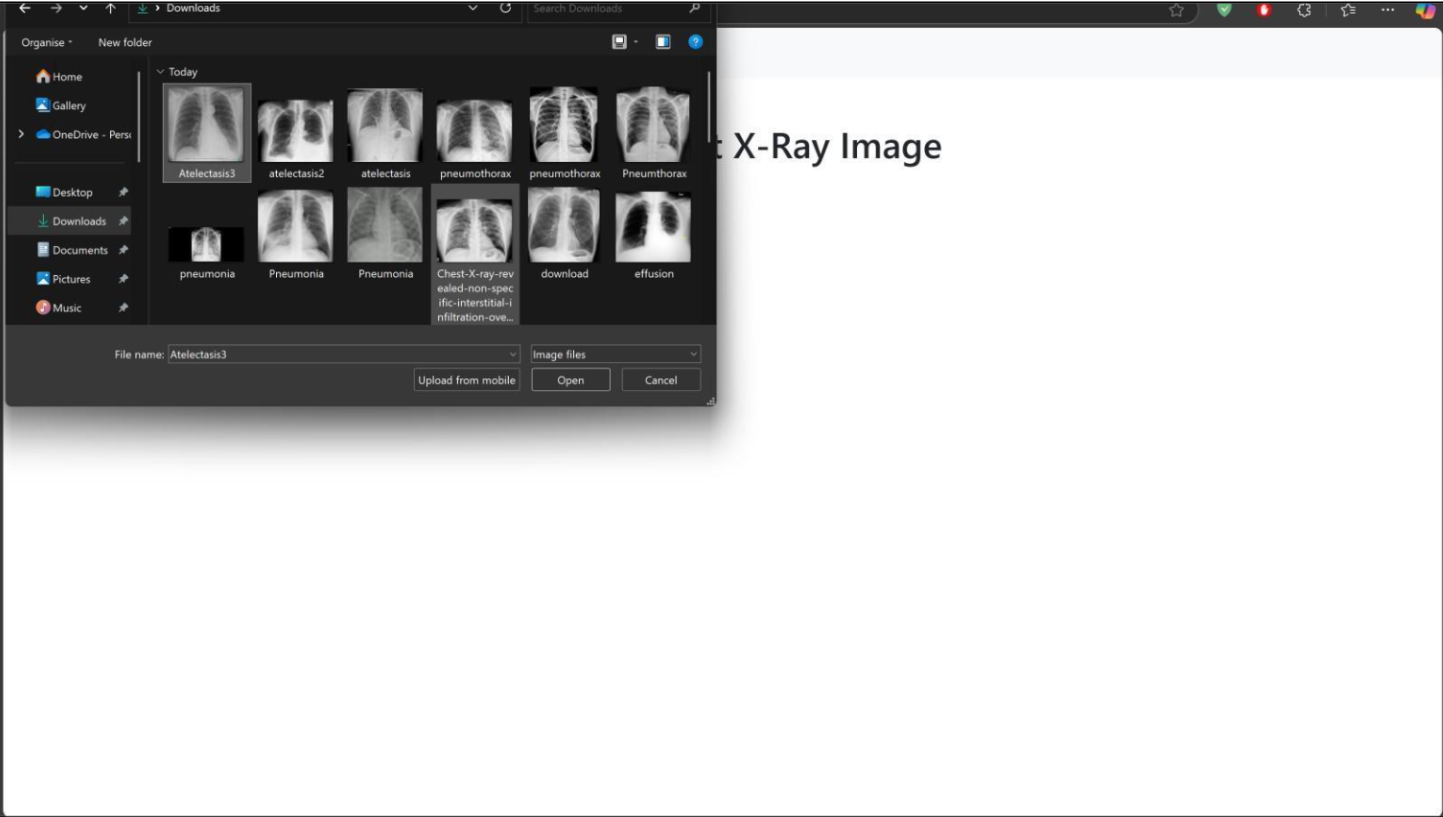
IV. LIMITATIONS
While the DenseNet121 model shows promising results for classifying chest X-rays, there are various limitations and challenges that must be tackled to ensure its effective use in clinical environments.
A. Overlap of Disease Manifestations:
Complexity of Medical Imaging: Chest X-ray images frequently show overlapping characteristics of various diseases, which complicates the model's ability to accurately differentiate between different conditions. For example, pneumonia and pneumothorax can display similar radiographic features, resulting in possible confusion in predictions (1, 6).
Model as an Assistant: Considering these challenges, the model should be seen as a support tool for healthcare professionals instead of a standalone diagnostic solution. It can assist in filtering and prioritizing urgent cases, which helps improve patient management efficiency.
B. Model Bias and Variance
Data Bias: The model's performance depends on the quality and distribution of the training data. If the dataset is biased or unbalanced, the model might adopt these biases, resulting in skewed predictions. Although the NIH Chest X-ray dataset used in this project is filtered to ensure high accuracy, there remains a chance of data errors and inconsistencies (3, 8).
Variance: The model can show significant variability, particularly when it encounters new or unfamiliar data. This may lead to changes in performance, underscoring the importance of ongoing assessment and refinement of the model.
C. Training Time and Scalability
Time-Consuming Training: Training deep learning models from the ground up takes a lot of time and computing power. When introducing new disease categories, the model needs to be retrained from the basics, which further extends the total training duration.
Scalability: The requirement for ongoing training sessions to incorporate new data and diseases can restrict the model's scalability. To address this challenge, effective strategies for incremental learning and model updates are crucial.
Even with these limitations, the DenseNet121 model proves to be a useful asset in the healthcare field. It aids healthcare professionals in identifying and prioritizing urgent cases, thereby improving diagnostic efficiency and contributing to better patient outcomes. Ongoing improvements in data quality, model training, and evaluation techniques will help address these challenges and promote the effective use of machine learning in clinical settings.
Conclusion
The use of machine learning in healthcare, especially with deep learning models such as DenseNet121, has shown great promise in improving diagnostic accuracy and efficiency. This project aimed to create a DenseNet121 model to classify chest X-ray images into four categories: Effusion, Atelectasis, Infiltration, and No Finding. A. Key Achievements 1) Data Preparation and Preprocessing: We effectively mapped image paths, cleaned up labels, and established binary columns for each condition. The data was thoroughly preprocessed to guarantee quality and consistency. 2) Model Design and Training: Using the DenseNet121 architecture with pre-trained weights from ImageNet, we fine-tuned the model specifically for chest X-ray classification. The training was conducted on a balanced dataset to minimize bias and improve overall performance 3) Evaluation: The model\'s performance was assessed through ROC curves and AUC scores, offering a thorough evaluation of its classification abilities. Although there were some difficulties with overlapping disease symptoms and data biases, the model showed strong performance, especially for Effusion and No Finding (1, 2). 4) User-Friendly Interface: The Flask application was improved to offer a more user-friendly interface, making it simple for users to upload images and see the classification results. Incorporating Bootstrap for styling enhanced the visual appeal and responsiveness of the web application.
References
[1] Rohit Kundu, et al. (2021). Pneumonia detection in chest X- ray images using an ensemble of deep learning models. PLOS ONE. [2] Mohammed A. A. Al-qaness, et al. (2024). Chest X-ray Images for Lung Disease Detection Using Deep Learning Techniques: A Comprehensive Survey. Archives of Computational Methods Engineering. [3] Various Authors. (2024). Machine learning and deep learning algorithms in detecting COVID-19. Springer. [4] Various Authors. (2021). COVID-19 detection using federated machine learning. PLOS ONE. [5] Siddhartha et al. (2023). Analyzing the Role of CNN Models in Healthcare Diagnostics. IEEE Transactions on Medical Image. [6] Uswatun Hasanah, Jenq-Shiou Leu, Cries Avian, Ihsanul Azmi & Setya Widyawan Prakosa (2024). A systematic review of multilabel chest X-ray classification using deep learning. Multimedia Tools and Applications. [7] V.Nagaraja & S.Rajasekaran (2024). Enhancing Chest X-Ray Image Classification Using Deep Learning With MobileNet And ResNet. IJCRT. [8] Ecem Sogancioglu, Erdi Çall?, Bram van Ginneken, Kicky G. van Leeuwen, Keelin Murphy (2021). Deep Learning for Chest X-ray Analysis: A Survey. arXiv. [9] Bram van Ginneken, et al. (2019). Boosted Cascaded Convnets for Multilabel Classification of Thoracic Diseases in Chest Radiographs. IEEE Transactions on Medical Imaging. [10] Various Authors. (2021). Comparison of Deep Learning Approaches for Multi-Label Chest X-Ray Classification. Multimedia Tools and Applications [11] Enhancing the Dataset: Incorporating more samples and diversifying conditions can improve the model\'
Copyright
Copyright © 2025 Prof. Priyanka Bhore, Utkarsh Kumar, Aniket Ovhal, Rahul Talvar, Nikhil Waghmare. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66707
Publish Date : 2025-01-27
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

